EVALUATION OF LEAD APRON AS A POTENTIAL SOURCE OF NOSOCOMIAL INFECTION IN OPERATING THEATER
Main Article Content
Abstract
Background: For radiation protection, lead aprons are often carried into the operating theater from the radiology department, where there are few or no dedicated lead aprons for the operating theater and can be a potential source of nosocomial infection.
Objective: The main objective of this research is to evaluate the presence of microorganisms on the lead aprons and devising a simple method of cleaning to lower the risk of contamination in the operating room.
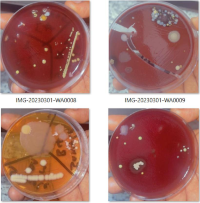
Methods: A total of 64 swabs were taken in two regions of the lead apron, the shoulders (inner and outer) and secondly, the front middle part of the apron (inner and outer). 40 samples were collected before cleaning, while 24 post-cleaning swabs was also taken from areas where growth were isolated using three different agents routinely used in our hospital: (cetrimide and chlorohexidine gluconate (savlon), isopropyl alcohol (methylated spirit) and sodium hydrochloride (Hypo/bleach). The samples collected were cultured on plates of Blood Agar, chocolate Agar, and MacConkey Agar and incubated at 37o c for 48h. The data was analysed using SPSS version 16.0, using descriptive statistics such as frequency and percentage.
Result: Growth was observed on 72.5% (n = 29) of X-ray aprons, and Staphylococcus aureus 69% (n = 20) and Staphylococcus epidermidis 31% (n= 9), were the pathogens observed on the lead apron, mostly on the outer part of the lead apron at the level of the patients and bed, followed by the inner part at the same level. The least noticeable area of growth is the inner part of the shoulder. After cleaning, no growth was detected in the 24 samples collected.
Conclusion: Lead aprons can be a potential source of nosocomial infections in the operating theater and cleaning them with any of the available disinfectants in the hospital before taking them to the room will significantly reduce the bacteria load.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
All articles in JRRS are published under the Creative Commons Attribution 4.0 International License (CC BY 4.0). This permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
How to Cite
References
1. Matinyi, S., Enoch, M., Akia, D., Byaruhanga, V., Masereka, E., Ekeu, I. Et al. Contamination of microbial pathogens and their antimicrobial pattern in operating theares of peri-urban eastern Uganda: a cross-sectional study. BMC infection Disease, 2018. 18:460.
https://doi.org/10.1186/s12879/-018-3374-4
2. Sikora, A., and Zahra, F. Nosocomial infection. Startpearls publisher. Treasure island. Treasure island (FL). 2021. Available at
https://www.cbi.nlm.nih.gov/books/NBK559312/
3. Khan, H. A., Kanwal, F. B. And Mehboob, R. Nosocomial infection: Epidemiology, prevention, control and surveillance. Asian Pac J of Tropical Biomedicine. 2017. 7(5): 1 – 5. Http://dx.doi.org/10.1016/j.apjtb.2017.01.019
4. Matthew, D., Dadah, A. J. And Mohammed, S. S. D. Bacterial Contamination of operating theatres: a case study of a hospital in northan Nigeria. Science world journal. 2020. 15(2): 84 – 90. ISSN 1597-6343.
5. Spagnolo. A. M., Ottria, G. Amicizia, D., Perdelli, F. And Christina, M.L. Operating theatre quality and prevention of surgical site infection. J. Prev Med Hyg. 2013. 54: 131-137.
6. Hold A. Infection control in theatre, Southern African Journal of Anaesthesia and Analgesia. 2011. 17(1): 56-64, DOI:
10.1080/22201173.2011.10872732) http://doi.org/10.1080/22201173.2011.10872732
7. Jaber, M. M., Qiao, E., Harvill M. L. And Nasser F. A. Contamination of interventional radiology lead apron by nosocomial pathogens. Biomed J Sci & Tech Res. 2017. 1(2). 391 -393. DOI: 10.26717/BJSTR.2017.01.000203
8. Raksha, G. S. Operation Theatres: A source of nosocomial infection. Era@s journal of Med. Res. 2019. 6(1): 1 - 6 Doi: 10.24041/ejmr2019.103.
9. Doll, M. Guide to infection control in the hospital: the new technologies in infection prevention. Chapter 56. international society for infectious diseases. 2018 1- 13. Available at https://isid.org/2018/02/ISID_infectionguige_chapter22.pdf
10. Okon, K.O., Osundi, S. Dibal, J., Ngbale, T., Bello, M., Akuwhwa, R. T., et al. Bacterial contamination of operating theatre and other
specialized care in a tertiary hospitals in northeastern Nigeria. Afr. J. Microbiol. Res. 2012. 6(13): 3092 – 3096. Doi: 10.5897/AJMR11.834
11. Kumar, P. Radiation safety issues in fluoroscopy during percutaneous Nephrolithotomy. Urol J. Winter. 2008. 5(1): 15 – 23. PMID 18454421 available at http://pubmed.ncbi.nlm.nih.gov/18454421/
12. Manhajan, A., Samuel, S., Saran, A. k. Mahajan, M. K. an Mam, M. K. Occupational radiation exposure from C arm Fluoroscopy During
common Orthopaedic Surgical Procedures and it prevention. J. clinical. Diagn. Res. 2015. 9(3): 1 –4. Doi: 10.7860/JCDR/2015/10520.5672.
13. Narain, A. S., Hijji, F. Y., Yom, K. H., Kudaravalli, T., Haws, B. E. And Singh, K. Radiation exposure and reduction in the operating
room: prospective and future directions in spine sugery. World J. Ofrthop. 2017, 8(7). 524 - 530 Doi: 10.5312/wjo.v8.i7.524
14. Kaplan, D.J., Patel, J.N., Liporace, F. A. And Yoon, R.S. Intraoperative radiation safety in orthopedics:a review of ALARA(as low as
reasonable achievable) principle. Patient saf surg. 2016. 10(27): 1 – 7. Http://doi.org/10.1186/s13037-016-0115-8
15. Boyle, H. and Strudwick, R. Do lead rubber aprons pose an infection risk? J. Radiography 2010. 16(4): 297-303. ISSN 1078-8174. available at http://www.radiographyonline.com/article/S1078-8174(10)00038-6/abstract
16. Bamanga, A., Umaru, A., Mohammed, Y. M., Musa, H. M., Mohammed, I. and Ibrahim, I. Z. Quality assurance of personal protective apparel at tertiary hospitals in Yola, Adamawa state. Journal of radiography and radiation sciences. 2022. 36(1). https://doi.org/10.48153/jrrs/2022/ZJYW8662
17. Jain, S., Rajfer, R. A., Melton-Kreft, R., Nistico, L., Miller M. C., Stoodley, P., et al. Evaluation of bacterial presence on lead X-ray aprons utilised in the operating room via IBIS and standerd culture methods. Journal of infection Prevention. 2019, 20(4):191-196 doi: 10.1177/1757177419833163
18. Feierabend, S. and Siegel G. Potential infection risk from thyroid radiation protection. Journal of orthopaedic trauma. 2015. 29(1). Doi: 101097/BOT.0000000000000161.
19. Muhammad, U. K., Alhaji Isa, M. and Aliyu, M. Z. Distribution of potential nosocomial pathogens isolated from enviroments of four hospitals in Sokoto. North western Nigeria. J. microbiol. biotech. Res. 2013. 3(1): 139 - 143. Available at http://www.researchgate.net/publication/296862219_distribution_of_potential_nosocomial_pathogens_isolated_from_enviroments_of_four_selected_hospital_in_sokoto_north_western_nigeria
20. Genet, C., Kibru, G. and Tsegaye, W. Indoor air bacterial load and antibiotic susceptibility pattern of isolate in operating room and surgical wards at jimmauniversity specialized hospital, southwest Ethiopia. Ethiop j health sci. 2011. 21(1): 9 – 17. doi: 10.4314/ejhs.v21i1.69039.
21. Percival SL, Emanuel C, Cutting KF, Williams DW. Microbiology of the skin and the role of biofilms in infection. International wound journal. 2012 Feb;9(1):14-32.
22. Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence?. British journal of dermatology. 2008 Mar 1;158(3):442-55.
23. Nagington J, Sutehall GM, Whipp P. Tonometer disinfection and viruses. British journal of ophthalmology. 1983 Oct 1;67(10):674-6.